
Wearable Medical Device
Industrial Design phase of this compact IPx4 wearable medical device started with researching into various dimensions. Product usability and user interaction was given utmost priority as the users are expected to wear this almost 24/7 for a period of 30 to 45 days. Part of usability study was to understand the adaptation of the device to most body types apart from consideration of body movements and finding the most suitable position for deployment.
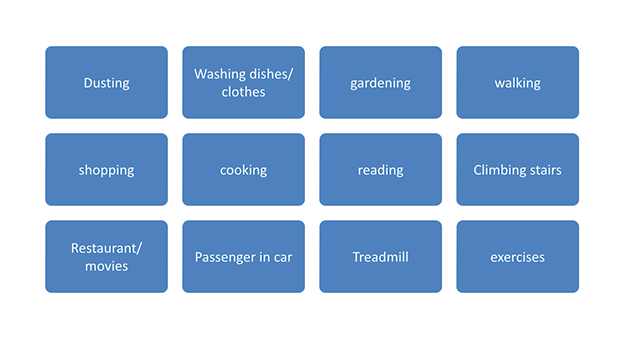
QMI (Quick Market Intelligence) was followed up with work study of typical user. Work study was done on a daily cycle as well as for a weekly cycle.

 Based on the research, various deployment approached were scrutinised.
Based on the research, various deployment approached were scrutinised.
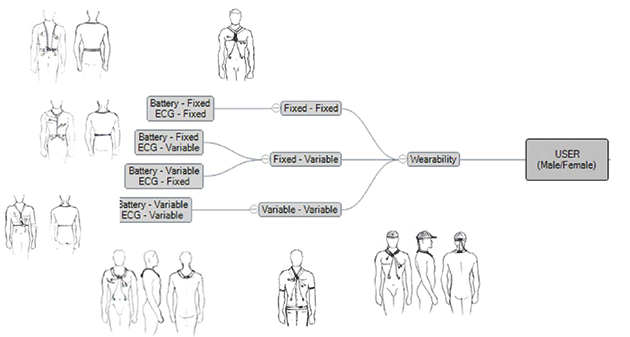
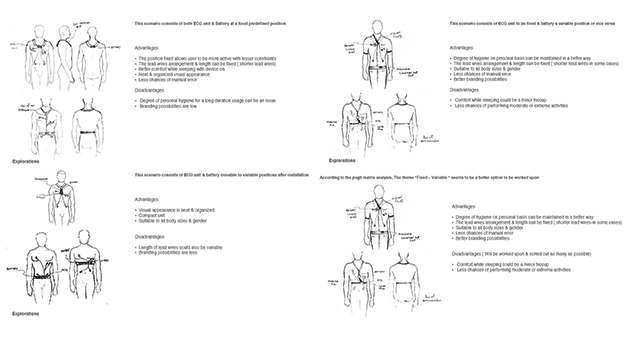 Two major approaches evolved as convincing, which was taken into more detail to arrive at the most promising approach. Another round of research was conducted into products available in the market for similar approaches focusing on how they performed. Key challenges were identified during the process.
Two major approaches evolved as convincing, which was taken into more detail to arrive at the most promising approach. Another round of research was conducted into products available in the market for similar approaches focusing on how they performed. Key challenges were identified during the process.
Quick mock-up were made to evaluate the volume of the product based on internal architecture.
Idea generation sketches were done followed by digital sketching.
Digital sketching were shortlisted to develop first lever 3D CAD models.
CAD models were further detailed to define features identified in the PDS (product design specification) Designs were prototyped to evaluate fit and finish, aesthetics, DFMA and BOM cost.
Designs were prototyped to evaluate fit and finish, aesthetics, DFMA and BOM cost.
View product launch video here 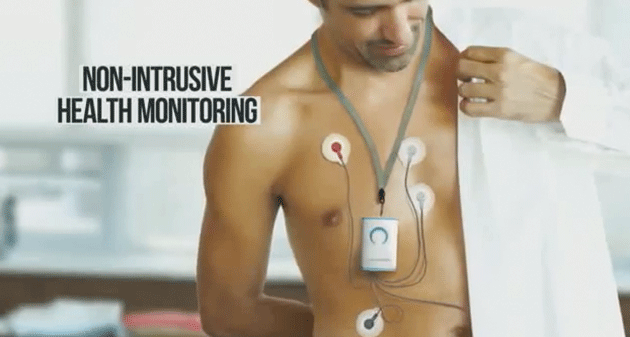
 Wearable medical device successfully completed CE certification.
Wearable medical device successfully completed CE certification.
End of Document.













